The mass density of a material varies with temperature and pressure. (The variance is typically small for solids and liquids and much greater for gasses.) Increasing the pressure on an object will decrease the volume of the object and therefore increase its density. Increasing the temperature of a substance (with some exceptions) decrease its density by increasing the volume of that substance.
Mathematically, density is defined as mass divided by volume:
The SI unit of kilogram per cubic metre (kg/m³) and the cgs unit of gram per cubic centimetre (g/cm³) are probably the most common used units for density. (The cubic centimeter can be alternately called a millilitre or a cc.) One g/cm³ equals 1000 kg/m³. In industry, other larger or smaller units of mass and or volume are often more practical and US customary units may be used. See below for a list of some of the most common units of density. Further, density may be expressed in terms of weight density (the weight of the material per unit volume) or as a ratio of the density with the density of a common material such as air or water.
http://en.wikipedia.org/wiki/Density
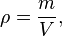
No comments:
Post a Comment